Research Paper: Generation of Aurachin Derivatives by Whole-Cell Biotransformation and Evaluation of Their Antiprotozoal Properties
Abstract:
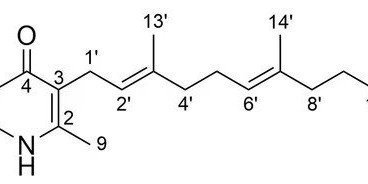
The natural product aurachin D is a farnesylated quinolone alkaloid, which is known to possess activity against the causative agent of malaria, Plasmodium spp. In this study, we show that aurachin D inhibits other parasitic protozoa as well. While aurachin D had only a modest effect on Trypanosoma brucei rhodesiense, two other trypanosomatids, T. cruzi and Leishmania donovani, were killed at low micromolar and nanomolar concentrations, respectively, in an in vitro assay. The determined IC50 values of aurachin D were even lower than those of the reference drugs benznidazole and miltefosine. Due to these promising results, we set out to explore the impact of structural modifications on the bioactivity of this natural product. In order to generate aurachin D derivatives with varying substituents at the C-2, C-6 and C-7 position of the quinolone ring system, we resorted to whole-cell biotransformation using a recombinant Escherichia coli strain capable of aurachin-type prenylations. Quinolone precursor molecules featuring methyl, methoxy and halogen groups were fed to this E. coli strain, which converted the substrates into the desired analogs. None of the generated derivatives exhibited improved antiprotozoal properties in comparison to aurachin D. Obviously, the naturally occurring aurachin D features already a privileged structure, especially for the inhibition of the causative agent of visceral leishmaniasis.
Das Paper "Generation of Aurachin Derivatives by Whole-Cell Biotransformation and Evaluation of Their Antiprotozoal Properties" ist zu finden unter https://www.mdpi.com/1420-3049/28/3/1066. Viel Spaß beim Lesen.





